Caslin Research Group
Our Work
Broadly, we work in the field of immunometabolism. This encompasses how immune cells regulate systemic metabolism and how bioenergetic pathways regulate immune cell function. Specifically, we are interested in how diet and exercise affect innate immune cells in health and disease.
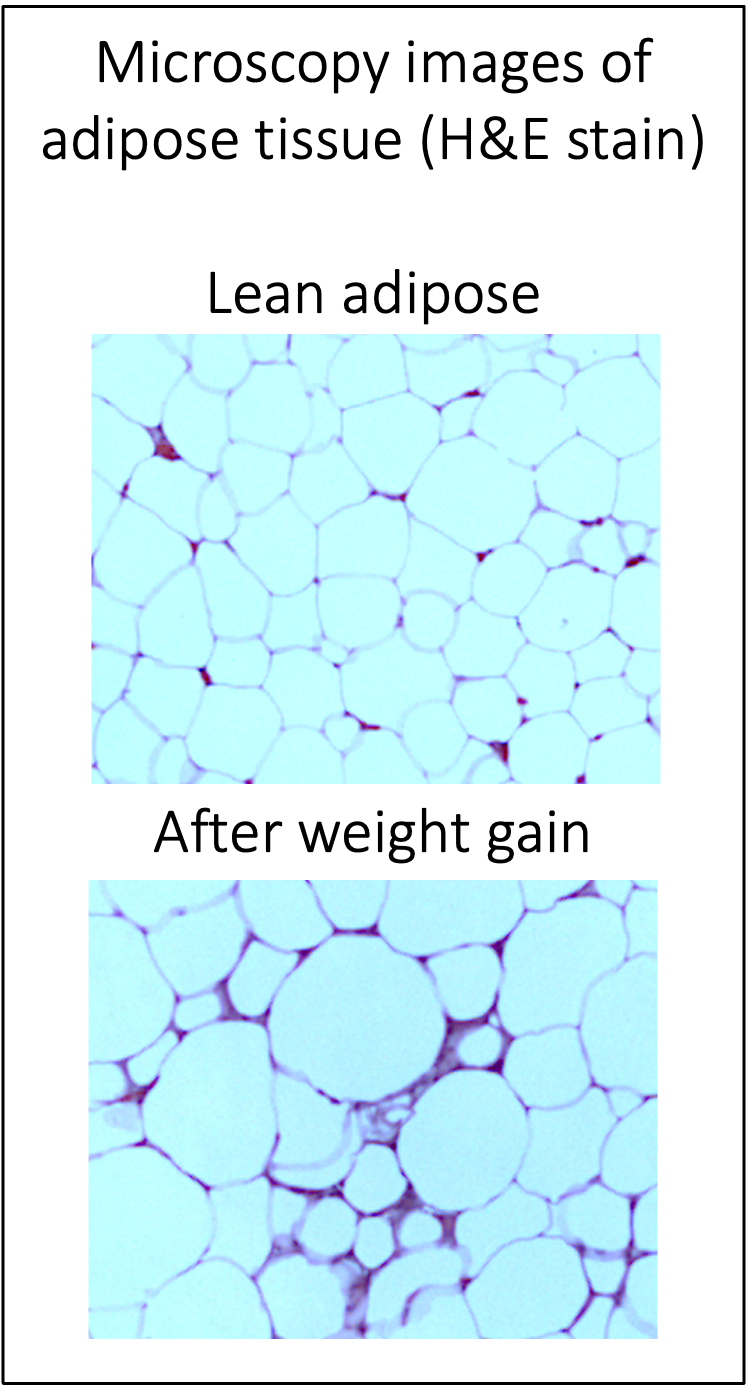
Adipose (fat) tissue is an important organ for energy storage, insulation, and hormone regulation; however, larger quantities of body fat are linked with the development of cardiovascular diseases and diabetes. Nearly all immune cells are found in the adipose tissue. Regulatory and “anti-inflammatory” immune cells reside in lean fat, while adipose expansion recruits and alters immune cells to become more “inflammatory”. Interestingly, many of the drugs used in the clinic for high cholesterol (statins) and diabetes (metformin) also have anti-inflammatory effects. Thus, we believe that adipose immune cells can be a useful therapeutic target for many metabolic diseases, and we hope to better understand how different diet, exercise, and therapeutic strategies can support adipose (and overall) immune health.

Current lab members
-
Heather Caslin, Faculty
-
Wishal Khan (PhD student)
-
Kingsley Oforka (co-mentored PhD student)
-
Munira Kapadia, Research Technician
-
McArthur Bolden, Research Technician
-
Emily Nguyen, Undergraduate Research Assistant
Current projects
1. Innate Immune Memory in Adipose Macrophages following Weight Cycling:
For many individuals with a high BMI/ degree of adiposity, weight loss is often prescribed in the clinic to reduce disease risk. However, weight loss is difficult to do and even more difficult to maintain. Importantly, weight regain is linked to a heightened risk for cardiometabolic diseases beyond the risk of obesity. In my postdoctoral studies, we found that while weight loss does improve diabetes risk in mice, adipose immune cells remain in their “inflammatory” state. Specifically, I found that weight loss can induce a “memory” to the state of obesity in adipose macrophages. We showed that while weight loss does improve diabetes risk, macrophages in the adipose tissue remain hypermetabolic (with higher glycolysis and oxidative phosphorylation) and hyperinflammatory (with greater secretion of inflammatory cytokines). We believe this heightened inflammation is beneficial to pathogen defense, but detrimental to metabolic disease, correlating with worsened diabetes risk upon weight regain.
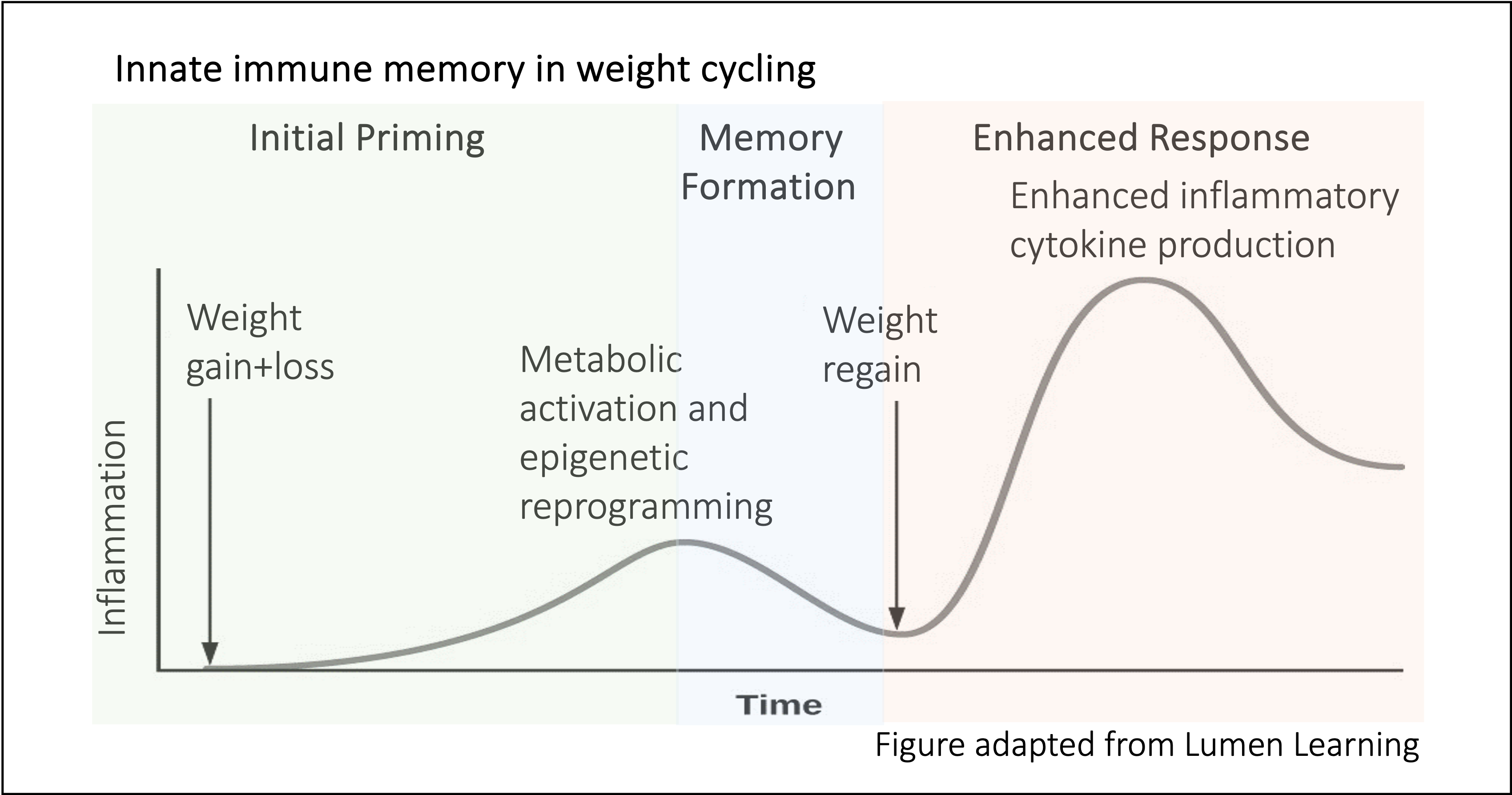
Currently, we are interested in understanding how innate immune memory forms in adipose macrophages during weight loss and if macrophage memory is the cause of worsened diabetes risk following weight regain. Additionally, we are interested to know if exercise can alleviate macrophage memory or diabetes risk with weight regain.
Related papers:
Caslin H.L, Cottam M.A., Piñon J.M., Boney L.Y. , Hasty, A.H. Weight cycling induces innate immune memory in adipose tissue macrophages. Frontiers in Immunology. 2023 Jan 11; 13. doi: 10.3389/fimmu.2022.984859.
Bolden III M., Davis X.D., Kapadia, M., Nguyen, E., Sherwood D.R., Bohannon J.K., Caslin H.L. Weight loss-induced adipose macrophage memory corresponds with lower adipose bacterial burden in murine systemic Staphylococcus aureus infection. Shock, Published ahead of print, March 2025. doi:10.1097/SHK.0000000000002575
Cottam M.A.*, Caslin H.L*, Winn N.C., Hasty A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes during weight loss and subsequent weight regain. Nature Communications. 2022 May 26;13(1):2950. doi: 10.1038/s41467-022-30646-4.
Caslin H.L*, Bhanot M.*, Bolus W.R., Hasty A.H. Adipose tissue macrophages: unique polarization and bioenergetics in obesity. Immunological Reviews. 2020; 00:1-13. doi: 10.1111/imr.12853.
Pilot Grant funding:
UH College of Liberal Arts and Sciences, Early Career Research Progress Grant (2024)
“Epigenetic Mechanisms of Adipose Macrophage Memory”
Houston Nutrition and Obesity Research Center, Pilot and Feasibility Award (2024-2025)
“Metabolic Mechanisms of Adipose Macrophage Memory”
American College of Sports Medicine, Early Career Research Grant (2024-2025)
“Does exercise alleviate weight cycling- accelerated metabolic disease and innate inflammation?”
2. Lipid-Associated Mast Cells:
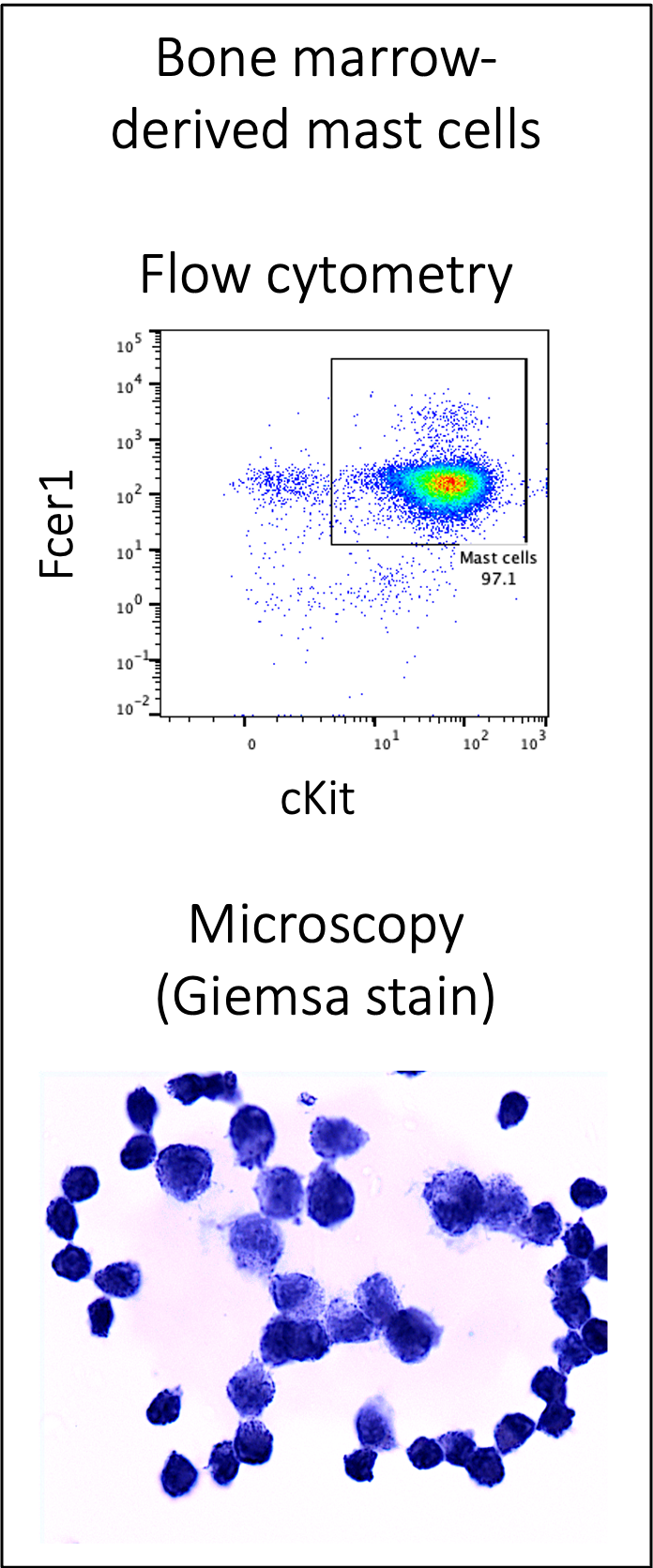
In my postdoctoral work, we also found that weight regain induces a novel lipid handling phenotype in adipose mast cells. These cells appear similar to lipid-associated macrophages that are present in the adipose tissue with one bout of weight gain. We are currently working to isolate this cell population from the adipose tissue, understand their development in culture, and understand their impact on diabetes risk following weight regain.
Related papers:
Caslin H.L., Cottam M.A., Betjemann A.M., Mashayekhi M., Silver, H.J., Hasty A.H. Weight cycling induces a novel lipid-associated mast cell population. bioRxiv. doi: 10.1101/2023.11.12.566786
Cottam M.A.*, Caslin H.L*, Winn N.C., Hasty A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes during weight loss and subsequent weight regain. Nature Communications. 2022 May 26;13(1):2950. doi: 10.1038/s41467-022-30646-4.