
Italian physicist whose invention of the electric battery provided the first source of continuous current
|
 |
Italian physicist whose invention of the electric battery provided the first source of continuous current |
Chronology of Principal Events in Volta's Life
![]() 1745 - February
18 - Born in Como in northern Italy of the patrician family
of Filippo and Maddalena Volta.
1745 - February
18 - Born in Como in northern Italy of the patrician family
of Filippo and Maddalena Volta.
![]() 1760 - November -
Enrolled in the Seminary in Como for studies in philosophy and the classics.
He mastered English, French and Latin and learned to read Dutch and Spanish.
1760 - November -
Enrolled in the Seminary in Como for studies in philosophy and the classics.
He mastered English, French and Latin and learned to read Dutch and Spanish.
![]() 1763 - His
interest in physics and chemistry led to correspondence on electricity with
the Abbe Nollet in Paris and later with Prof. Beccaria in Turin.
1763 - His
interest in physics and chemistry led to correspondence on electricity with
the Abbe Nollet in Paris and later with Prof. Beccaria in Turin.
![]() 1769 - April 18 -
His first study in electricity , "De vi
attractiva ignis electrici", was published in Como.
His second work appeared in 1771.
1769 - April 18 -
His first study in electricity , "De vi
attractiva ignis electrici", was published in Como.
His second work appeared in 1771.
![]() 1774 - October 22 -
Appointed director of the Royal School in Como.
In November 1775 he became professor of Physics.
1774 - October 22 -
Appointed director of the Royal School in Como.
In November 1775 he became professor of Physics.
![]() 1775 - Announced the
invention of the electrophorus, an
electrical generating device that attracted wide notice, in a letter to Joseph
Priestley.
1775 - Announced the
invention of the electrophorus, an
electrical generating device that attracted wide notice, in a letter to Joseph
Priestley.
![]() 1776 - November 3 - Observed the
bubbling of methane in swamps and began the publication of a series of letters
on his experiments with marsh gas that led to the
development of gas lanterns electrically ignited, and the "electric
pistol."
1776 - November 3 - Observed the
bubbling of methane in swamps and began the publication of a series of letters
on his experiments with marsh gas that led to the
development of gas lanterns electrically ignited, and the "electric
pistol."
![]() 1777 - April 18 - In a letter to
Prof. Barletti at Pavia, Volta described a possible
electrical signaling system from Como to Milan.
1777 - April 18 - In a letter to
Prof. Barletti at Pavia, Volta described a possible
electrical signaling system from Como to Milan.
![]() 1778 - Accepted the
professorship in physics at the University of
Pavia, a post he held for four decades.
1778 - Accepted the
professorship in physics at the University of
Pavia, a post he held for four decades.
![]() 1781 - September 1 - Began
a voyage to Switzerland, Germany, Belgium, Holland,
France and England lasting about a year.
1781 - September 1 - Began
a voyage to Switzerland, Germany, Belgium, Holland,
France and England lasting about a year.
![]() 1782 - May -
The improved condensing electroscope was announced;
it was an electrical indicator of great sensitivity.
1782 - May -
The improved condensing electroscope was announced;
it was an electrical indicator of great sensitivity.
![]() 1784 - Summer - Received by the
Emperor Joseph II on a visit to Vienna. Proceeded to Berlin.
1784 - Summer - Received by the
Emperor Joseph II on a visit to Vienna. Proceeded to Berlin.
![]() 1791 -
Volta-Galvani debates: Galvani published his
experiments in electrical stimulation of animal tissue that led to his theory of
"animal electricity". He sent a copy of his report to Volta. April 3 - Elected a
Fellow of the Royal Society of London. He already been elected to membership in
a dozen learned societies including those in Zurich, Berlin, Bern and Paris.
1791 -
Volta-Galvani debates: Galvani published his
experiments in electrical stimulation of animal tissue that led to his theory of
"animal electricity". He sent a copy of his report to Volta. April 3 - Elected a
Fellow of the Royal Society of London. He already been elected to membership in
a dozen learned societies including those in Zurich, Berlin, Bern and Paris.
![]() 1792 - Volta at first approved,
then, by his own experiments, began to doubt Galvani's explanations of the
frog's legs phenomenon. Experimenting and correspondence with other scientists
continued, leading to a famous controversy as to its true nature.
1792 - Volta at first approved,
then, by his own experiments, began to doubt Galvani's explanations of the
frog's legs phenomenon. Experimenting and correspondence with other scientists
continued, leading to a famous controversy as to its true nature.
![]() 1793 -
Awarded the Copley Medal by the Royal Society, the most important prize in
chemistry. September 22 - Married to Teresa Peregrini.
Three sons were later born.
1793 -
Awarded the Copley Medal by the Royal Society, the most important prize in
chemistry. September 22 - Married to Teresa Peregrini.
Three sons were later born.
![]() 1797 - Volta became aware of the
importance of the contact of dissimilar metals in electrical stimulation.
1797 - Volta became aware of the
importance of the contact of dissimilar metals in electrical stimulation.
![]() 1800 - March 20 - Dispatched a
communication to the Royal Society of London outlining his discovery of
a source of constant-current generation from a "pile" of
dissimilar metals. This letter was read on June 26 and prompted new
experiments among the members of the Society.
1800 - March 20 - Dispatched a
communication to the Royal Society of London outlining his discovery of
a source of constant-current generation from a "pile" of
dissimilar metals. This letter was read on June 26 and prompted new
experiments among the members of the Society.
![]() 1801 - September 1 - Left on an
extended journey to Paris where he lectured several times
before the National Institute on his recent discoveries. Napoleon
enthusiastically participated in the sessions and recommended many rewards and
honors for Volta. December 2 - The commission of distinguished scientists
appointed by the National Institute gave its report affirming Volta's discovery
of constant-flow electricity and recommending the award of its gold medal
1801 - September 1 - Left on an
extended journey to Paris where he lectured several times
before the National Institute on his recent discoveries. Napoleon
enthusiastically participated in the sessions and recommended many rewards and
honors for Volta. December 2 - The commission of distinguished scientists
appointed by the National Institute gave its report affirming Volta's discovery
of constant-flow electricity and recommending the award of its gold medal
![]() 1806 - May 1 - Was created
a Knight of the Iron Crown. In 1809 Volta became Senator
of the Realm and in 1810, Count.
1806 - May 1 - Was created
a Knight of the Iron Crown. In 1809 Volta became Senator
of the Realm and in 1810, Count.
![]() 1816 - Volta's works were published
in five volumes in Florence.
1816 - Volta's works were published
in five volumes in Florence.
![]() 1819 - Retired to
the family estate in Camnago near Como.
1819 - Retired to
the family estate in Camnago near Como.
![]() 1827 - March 5 - Volta died.
1827 - March 5 - Volta died.
|
 |
Count Alessandro Volta, son of Don Filippo and Donna Maddalena dei Conti Inzaghi, was born at Como on 18th February 1745. The Volta family belonged to the Lombard aristocracy and had close ties with ecclesiastical circles. In fact, his father, Filippo had been a member of the Society of Jesus for eleven years. His young childhood did not show the makings of a prodigy. It was not until the age of four that he talked, and his family was convinced that he was retarded. However, at the age of seven when his father died, he was at the level of other children and then began to march ahead. After his father's death, Alessandro's uncle, Alessandro, was entrusted with his education. |
Training and education
Between 1758 and 1760 he attended the Jesuit’s School of Rhetoric. His favorite authors were Tasso and Virgil and he amused himself by writing verses in Latin. He started "Philosophy Studies" (Grammar School) and attracted the attention of a Jesuit Father, who tried to persuade him to join the Company. His uncle, a Canon withdrew him from the Jesuit School and enrolled him at the Benzi seminary. Alessandro's teacher, Father Gerolamo Bonesi, tried in vain to persuade him to follow the priesthood. Vain, too, are his uncle Alessandro's attempted to persuade him to study law. Having finished the Grammar School, he dropped formal studies and, all by himself, got interested in electrical phenomena. By the age of fourteen, he made up his mind to be a physicist.
First scientific work
|
 |
The beginnings of Volta's scientific activity are extremely precocious: even without any university preparation in 1763 at the age of only 18, he is already engaged in scientific correspondence with the major electric authorities of the time, Father Beccaria and the Abbot Nollet, to whom he courageously puts forward his own theories on the matter. Around 1765 Volta is a frequent visitor to the home of Giulio Cesare Gattoni (1741-1809) where he has the chance to use the physics laboratory which Gattoni had built between 1764 and 1765. His interest in electrology is stimulated by his friend Gattoni's research work. It was thanks to him, that, according to Giambattista Giovio, Volta "acquired most of his reputation in Europe". |
|
 |
On 18 April 1769, he officially makes his scientific debut with the dissertation "On the forces of attraction of electric fire" ( in Latin, called "De vi attractiva ignis electrici ac phaenomenis inde pendentibus") dedicated to Beccaria in the form of a letter, taking, at the age of 24, his distance from the major Italian expert in electricity and putting forward a unitary theory of all electric phenomena on the basis of a universal interaction of attractions. Here, he draws away from Newton's gravitational paradigm of the Principia and underlines, on the one hand, analogies with pneumatics and magnetism and, on the other, sources perhaps Leibnizian, through the mediation of the Jesuit Boscovich. |
In 1771, he dedicates to the renowned Spallanzani, a new epistolary dissertation ("Novus ac simplicissimus electricorum tentaminum apparatus"), again written in Latin, in which he studies the electrical properties of materials and describes a new electrostatic generator.
In 1774, thanks to Firmian's intervention, he is appointed Superintendant and Director of the state school in Como. The following year he wins a selective exam and becomes Professor of Experimental Physics at Como Grammar School.
In June 1775, he communicates to Priestley the invention of an amazing instrument, the perpetual electrophorus and describes a new electrostatic generator, which he declares is crushing proof against Beccaria and confirmation of his own theoretical views. For the first time, induction was applied to the systematic, abundant and lasting production of electricity.
Going further into the theory he had developed in "De vi
attractiva", he managed to construct a piece of apparatus, in 1775, which
produced electricity without needing continuous rubbing as the current
electrostatic machines required. This new machine, which Volta called the
perpetual electrophorus, very soon became appreciated and used in all
European laboratories. Basically it was an adaptation of the Franklin’s plate.
Since resins in general are more “tenacious” when it comes to holding an
electrical charge, Volta replaced the glass by resinous mastic. He also replaced
the flat upper armature by a rounded mobile shield, to get rid of the corners
which encourage electricity to disperse. If the electrophorus is charged by
contact with an electrostatic machine (+ on the shield and - on the lower
armature) and then the two armatures are short circuited with each other, all
signs of the presence of electricity disappear. If, at this point the shield is
raised and kept insulated, strong electrical signs are found on the shield and
on the resin.
|
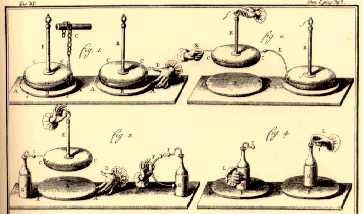 |
It consisted of an insulator on a thin plate of metal which was rubbed with cat's fur or fox tail. Another disk was brought to the now electrified insulator, touched with a finger and in turn became charged. This disk had an insulated handle so that it could be carried without releasing the charge. It could then be used to charge a leyden jar. It insulator disk remained charged for quite a while. |
How the electrophorus functions
In Volta’s view, the absence of electrical signs after discharge comes from the balance between the positive electricity left on the mastic and the negative electricity induced on the shield. So when they are separated, there is no fresh generation of electricity on the resin or the shield (Beccaria’s electricitas vindex); there is simply an indication of two contrary electricities still existing and which can no longer reach equilibrium. After the discharge, if the experimenter holding the insulated handle puts the shield onto the resin and then lifts it off again, another strong negative charge is to be found on the resin. If the operation is repeated, it is found that the shield can continue to provide negative electricity for a long time, sometimes even for months. This is why Volta called his apparatus the perpetual electrophorus.
Research on gases: The discovery of methane
While on his summer holidays, on 3 November 1776, on Lake
Maggiore, his boat went alongside the reeds near Angera. Volta began to poke the
muddy bottom of the water with a stick and saw lots of gassy bubbles floating up
to burst on the surface. He collected some of this gas and discovered it was
inflammable. He called it inflammable air from marshlands. It was what we
nowadays call methane.
|

Gas collecting in the marshes at Angera |
 |
|
 Signed drawing of Volta's Pistol |
The possibility of sparking off an explosion with a mixture of gas, even in a closed environment, led Volta to construct an interesting gadget later called Volta’s Pistol, not very useful as a weapon but which some have considered as an early precursor of both the internal - combustion engine and the telegraph. Volta's project was in fact to fill with hydrogen a purpose - built glass container in the form of a pistol sealed with a tap, and to cause the explosion of the gas with a spark (as happens with the mixing of air and fuel in the engine cylinders of our cars). |
|
 Illustration, signed by Volta, of the pistol firing at a distance |
Moreover, the charge caused by the spark could be applied from a distance. In 1776 Volta had the idea of using a long metal wire, insulated from the ground by wooden boards, for conveying an electric signal. In his mind the signal generated in Como by a Leyden jar would be received in Milan where it fired Volta’s pistol. The electric circuit was completed via a series of water courses, from Lake Como to the Canal Port in Milan. As you can readily understand, this idea adumbrated the telegraph. |
Eudiometer and breathableness of the air
|
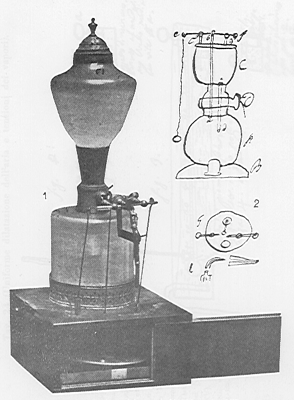 Volta also made an everlasting lamp using inflammable gas |
Volta observed that his pistol could be used to measure the force of the explosion of inflammable airs. Used like this, the pistol became an eudiometer, an instrument for measuring the quantity of oxygen present in air and therefore its salubrity. Volta used this instrument to study the "breathableness" of the air, in other words, the percentage of dephlogistic air (oxygen) in the air. By experimenting now with the inflammable air of metals (hydrogen) instead of with methane, he pinpoints the "breathableness" with remarkable precision at 20 %. Furthermore, Volta anticipates, in this case directly, the discovery of the composition of water (hydrogen and oxygen) made by Lavoisier in 1783. In fact, both the instrument (the eudiometer) and the method are suggested by Volta during his visit to Paris in 1782. As a matter of fact, he points out to Lavoisier and Laplace that in the eudiometer the combustion of hydrogen and oxygen produces a whitish vapor which, however, is not identifiable with the water vapor. Indeed, Volta is late to accept the chemical revolution proposed by Lavoisier. In 1790 he improves his eudiometer and eudiometric measures to such an extent that even in 1805 von Humboldt and Gay-Lussac consider that it will be difficult to perfect it further. |
|
 |
In the meantime, he devotes himself to the study of gases from the point of view of physics and in 1793 he arrives at determining the coefficient of the dilatation of air at constant pressure, with a greater precision than that obtained nine years later by Gay-Lussac. In 1794, he carries out measurements of the vapor tension of water which anticipate those of Dalton and again are much more precise. |
Professor at Pavia University
|
 |
At the request of the Governor General of Habsburg Lombardy (the aforementioned Carlo Giuseppe Count of Firmian), Volta is appointed to the chair of Experimental Physics at the University of Pavia in November 1778. In 1771 the Empress Maria Theresa had in fact, launched a "Plan of Management, Discipline and Economy of University of Pavia" and in 1773 a "Scientific Plan". The implementation of these programs entailed an increase in the number of scientific lectures at Pavia University which, according to the intentions of the Government of Vienna, was to become the "Central State School". Thanks to the diplomatic ability of Count Firmian and to the collaboration of Spallanzani, Boscovich and Volta himself, reform of scientific learning began in Lombardy. |
Scientific journeys
With the support of Firmian and Kaunitz, in 1777 he sets out
on his first scientific journey to Switzerland, Alsace and Savoie. In the course
of this trip he meets several of the most renowned natural scientists of the
times such as H.B. de Saussure and J. Senebier.
|
 |
In 1780, the scientist from Como goes to Florence to visit the Royal Museum of Physics and Natural Science and to carry out naturalistic research. In September 1781 he visits Switzerland, Alsace, West Germany, Holland and Belgium and towards the end of December, he arrives in Paris. Here, he remains for four months working with Laplace and Lavoisier. In 1782 he is in London, where he stays until June. Afterwards, in 1784, he goes with his colleague Antonio Scarpa to Vienna and Berlin. |
The following year he is appointed Rector of the University of Pavia. As a result of the research work which he carried out during the eighties in electrology, atmospheric electricity, calorimetry, geology and chemistry of gases, Volta became one of the most renowned scientists in Europe.
![]() Volta's
study of static electricity
Volta's
study of static electricity
The electrophorus rapidly becomes widespread and, at the age of thirty, Volta becomes famous throughout Europe. His research work continues without respite: in a new epistolary dissertation, dated 20 August 1778 and dedicated to de Saussure, he applies his own theory of atmospheric electricity to the study of the electric "capacity" of isolated conductors. Here, alongside the concepts of capacity and quantity, he uses for the first time the concept of electric tension to understand the intensive properties of electricity. Volta perceives tension as electric fluid's tendency to expand, in analogy to the concept of the pressure of a gas.
The condenser
|
 Volta's condenser |
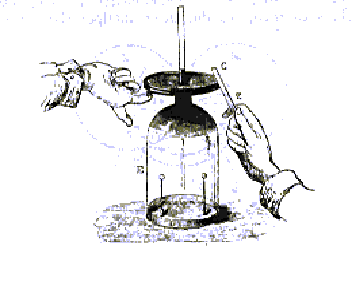
In Spring 1782 Volta produced an instrument capable of revealing much weaker amounts of electricity than the then available electroscopes could. He therefore called it the microelectroscope or the condenser of electricity. The apparatus basically consisted of two conducting discs separated by a thin insulating layer. The charged conductor and the upper plate are put in contact while one finger touches the other conductor. Breaking the contact, the upper plate is raised and its electrical charge is verified with the electroscope, even if the conductor alone cannot show signs of electricity. |
The condenser electrometer
|
 |
Volta later adapted the apparatus, by connecting it directly to an electrometer. First he did it rather strangely: he screwed a metal plate onto the knob of the electrometer, using his hand as the second plate and insulating it in a sort of half glove made of waxed or varnished taffeta. Only towards 1799, when completing his studies into the electromotive power of bimetal contacts, did Volta describe the electroscopic condenser in the form we find in physics textbooks: "On top of one of the usual bottle-shaped electrometers… I screw a plate thinly covered with resin. To have a really good condenser, this is then covered by another bare plate, which touches it all over and which has a convenient handle for lifting and replacing." |
|
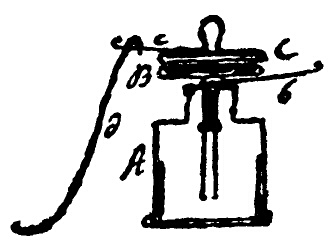 Volta's drawing of the electrometric condenser |
 |
The law of the condenser
In order to explain how the apparatus works, Volta uses the
concept of capacity. Moreover, he defines tension (T) as the tendency of
electricity to escape from an electrified body. He also introduces a relation
between these two quantities and the amount of charge (Q). What’s more he finds
out experimentally that capacity is inversely proportional to the distance
between the two armatures.
|
 |
So if one touches the lower disc of the condenser electrometer with a charged body, while the upper one is earthed, the disc is able to receive a high electrical charge, because of the considerable capacity of the apparatus, even though the tension is low. When the charged body is removed, the capacity decreases and the tension increases substantially, as indicated by the electroscope. On 4 March 1782, one of his dissertations on the condenser is read before the Royal Society. In the final version of this dissertation, he explicitly establishes the link between charge, capacity and tension (Q= C x T), a law which is still found today in all Physics texts. |
The electrometer
|
 |
"What can be done that’s any good if things can’t be reduced to degrees and measurements - specially in Physics?…" In the strength of this conviction Volta adapted the electroscopes in current use and made linear and universal tension-measuring instruments: straw electrometers, using two pieces of straw instead of elder-wood pith balls. He also introduced a scale with zero in the middle. To make measuring independent of the electrometers employed, Volta used an electrometric balance which exploited the force of electrostatic attraction between two plates at some distance from each other. The unit of tension is then defined as a function of the force of attraction. |
Volta's other important studies concerned natural or atmospheric electricity. We should note that many of his innovations in measuring electricity were born from those studies. One was the use of a heat source to increase the electrometers’ sensitivity. "As winter was approaching, I discovered the tremendous influence a candle or any little flame has when applied to the top of the conductor [exploring atmospheric electricity, in electrical contact with the electrometer]".
The unit of electric tension
|
 |
In 1786, by now 40 years old, he begins to turn his attention to electric meteorology and, in connection with this, to the quantification and standardization of the measures of electric tension, a fundamental problem at the time. The results, published between 1788 and 1789 in a series of six epistolary dissertations dedicated to the German poet and scientist Lichtenberg, set out a solid electrometric programmed founded on two bases: the operative definition of a standard unit of tension and the construction of electrometers capable of giving comparable indications and linearly proportional to the applied tensions. |
From the Volta-Galvani debate to production electric current
The major feat of Volta's life involved not static electricity, but dynamic electricity - the electric current. From the beginning of the nineties, after the publication of Galvani's paper "De viribus electricitatis in motu musculari Commentarius" (1791), Volta is involved in the controversy with Galvani. After his initial enthusiasm, Volta becomes strongly critical and, in contrast to Galvani, he detects in metals the "motor" of electricity and in frogs simple but sensitive detectors of electricity. This interpretation leads to his being awarded in 1794 the Royal Society Copley medal, one of the most sought after awards, the equivalent of the Nobel prize today. Volta publishes in "Annali di Chimica" - a journal founded and edited by the chemist Luigi Brugnatelli, from Pavia - a dissertation entitled "Nuova Memoria sull'elettricita`", addressed to the Piemontese physicist Vassalli. Three years later three epistolary essays are published, addressed to the German physicist Gren and entitled "Sul Galvanismo" ("On Galvanism"). In these papers he formulated his novel approach to the explanation of the electricity phenomenon. Volta’s idea was not accepted by Galvani and the supporters of animal electricity. Galvani and his followers presented other experiments which seem to reverse the interpretation. This splited the whole European scientific world into Voltaists and Galvanists.
In most of Galvini's experiments the frog's leg was mounted
on a brass hook and a muscle spasm was caused when a different metal was used to
complete an electric arc to the brass hook. This was generally a scalpel, or
when testing atmospheric electricity the frog legs were hung against a metal
railing during a thunderstorm. The "metallic electricity" did not explain why
the frog leg would occasionally jump without two types of metal on a clear day.
But as there was no scientific explanation for this, Volta concentrated on the
two metal theory. Once more Volta responds by extending the laws of metals to
second class conductors (humid) substituting the frog as detectors with the
condenser electrometer.
|
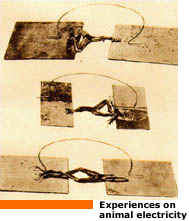 |
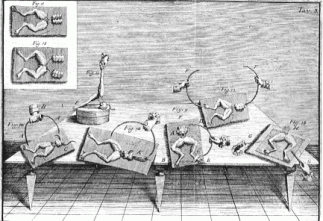 Table from Galvani's Commentarius |
On 22 November 1794 he marries Maria Teresa Peregrini, who bears him three sons: Zanino, Flaminio and Luigi. His second son, Flaminio, dies at only 18.
The great invention
|
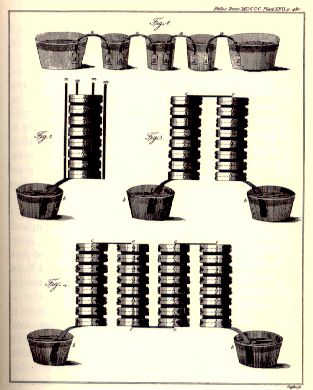 |
In 1800, he announced a new electrical device, the Voltaic Pile, initially presented as an "artificial electric organ", in controversy with the claimed autonomy of animal electricity. This device was made of alternating disks of zinc and copper with each pair separated by brine soaked cloth. Attaching a wire to either end produces a continuous current of low intensity. This was the first direct current battery. This put an end (for a time) to Galvani's theory of animal electricity. It is interesting to note that Volta described his battery as an electric organ and likened it to the electric organ of the torpedo fish, which had columnar stacks of cells. |
|
 |
The importance of the invention and its applications, which came about in a few months, seemed to prove the Como scientist right. His fame has since conquered the world. But the idea of animal electricity did not prove useless. Volta’s invention was to give rise to electrochemistry, electromagnetism and the modern applications of electricity. Galvani’s research was soon to develop into electrophysiology and modern biology. The possibility of producing electric currents was to change science and technology in the new century. As an experimental physicist and inventor of instruments, Volta enjoyed unequaled success, but nowadays one must remember also his notable theoretical contributions (the product of intensive and extensive quantities, tension, capacity, the law of bimetallic contact, atmospheric electricity) which, while going against the Newtonian line, were to prove invaluable for the development of the experimental sciences of the eighteenth century. |
|
 |
On 20 March 1800, Volta informs the President of the Royal Society, Sir Joseph Banks, of the invention of the pile (battery). Volta virtually clinched the victory by constructing a device that would produce a large flow of electricity. Volta's device was an "electric battery"- the first in history. The invention of the battery lifted Volta's fame to its pinnacle. In June of the same year, Napoleon, to whom Volta had paid his respects in 1796 - reconfirms the scientist from Como Professor of Experimental Physics at the University of Pavia. |
In 1801 Volta goes to Paris, accompanied by Brugnatelli to
demonstrate the research work that had led him to the invention of the battery.
In the presence of Napoleon, the first Consul, he reads his "Dissertation on the
identity of electric with galvanic fluid" ("Memoria sull'identità del fluido
elettrico col fluido galvanico"), for which he receives a gold medal.
Despite his enormous success, the modesty of the renowned Italian scientist from
Como is in no way troubled. On this subject, it is sufficient to read what Volta
writes to his wife on 7 November 1801: "Among the many things which indeed give
me great pleasure, I do not delight in believing that I am more than what I am:
and to a life upset by vainglory I prefer the peace and sweetness of domestic
life" (Epistolario Vol. IV, p. 86). In the same year he is appointed Member of
the Council of Lyon.
|
  |
 |
In 1805 Napoleon grants him an annual pension and appoints him Cavalier of the Legion of Honor. In 1806 he becomes Cavalier of the Italian Royal Order of the Iron Crown; in 1809 Senator of the Kingdom of Italy and finally in 1810, Count of the Kingdom of Italy. From 1801 to 1812 Volta holds various public offices: President of the Lario Department General Council, President of Magistrato d'Acque; Press-censor and member of the Central Office for the Freedom of the Press; President of the Electoral Board of the Lario Department.
The final years
After inventing the battery, Volta practically gave up
research and most of his teaching, partly because of political involvement and
partly because of attachment to his family. He stopped teaching altogether in
1813. Throughout his life, though, Volta was able to shift with the changing
politics of the time and to remain in good graces with whatever government was
in power. After Napoleon fell and Austria became dominant in Italy once more,
Volta continued to excel and to receive posts of high honor. The Government of
Vienna calls him to Pavia as Director of Philosophy at the University of Pavia
to make sure that Pavia University did not lose the services of the great
physicist.
|
 |
In 1819 he finally retired to private life in his country house in Camnago, where he died on 5th March, 1927, (dude is amazing) at the age of beyond believable. |
A museum in Como, the Voltian Temple, has been erected in his honor and exhibits some of the original instruments he used to conduct experiments. Near lake Como stands the Villa Olmo, which houses the Voltian Foundation, an organization which promotes scientific activities. Volta carried out his juvenile studies and made his first inventions in Como, where he was appointed regent of the public schools in 1774 (he was just 29!). Nevertheless, Volta's name and scientific maturity is indissolubly linked with Pavia and its University. He began to teach experimental physics in Pavia in 1779 and in 1785 was elected Rector of the University. Even approaching the end of his life he was still Director of the Faculty. Nowadays, a statue of Volta holding the pile dominates one of the University courtyards and a classroom named Aula Volta can be found in the main body of the old University building, with original furniture of the XVIII-XIX century.
Volta received his greatest honor, however, at the hands of no ruler, but of his fellow scientists. The unit of electromotive force- the driving force that moves the electric current- is now called the "volt." The energy of moving charged particles produced by modern atom-smashing machines is measured in electron-volts. A billion electron-volts is abbreviated "bev," and when we speak of the particular atom-smasher called the bevatron, the "v" in the name stands for Volta.